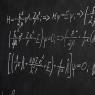The theory of the structure of organic compounds position. Theory of the structure of organic compounds
How science took shape at the beginning of the 19th century, when the Swedish scientist J. Ya. Berzelius first introduced the concept of organic substances and organic chemistry. The first theory in organic chemistry is the theory of radicals. Chemists discovered that during chemical transformations, groups of several atoms pass unchanged from a molecule of one substance to a molecule of another substance, just as atoms of elements pass from molecule to molecule. Such “immutable” groups of atoms are called radicals.
However, not all scientists agreed with the radical theory. Many generally rejected the idea of atomism - the idea of the complex structure of a molecule and the existence of an atom as its component part. What has been indisputably proven today and does not raise the slightest doubt, in the 19th century. was the subject of fierce controversy.
Lesson content lesson notes supporting frame lesson presentation acceleration methods interactive technologies Practice tasks and exercises self-test workshops, trainings, cases, quests homework discussion questions rhetorical questions from students Illustrations audio, video clips and multimedia photographs, pictures, graphics, tables, diagrams, humor, anecdotes, jokes, comics, parables, sayings, crosswords, quotes Add-ons abstracts articles tricks for the curious cribs textbooks basic and additional dictionary of terms other Improving textbooks and lessonscorrecting errors in the textbook updating a fragment in a textbook, elements of innovation in the lesson, replacing outdated knowledge with new ones Only for teachers perfect lessons calendar plan for the year; methodological recommendations; discussion program Integrated LessonsTopic: Basic principles of the theory of the structure of organic compounds by A. M. Butlerov.
The theory of the chemical structure of organic compounds, put forward by A. M. Butlerov in the second half of the last century (1861), was confirmed by the works of many scientists, including Butlerov’s students and himself. It turned out to be possible on its basis to explain many phenomena that had not yet been interpreted: homology, the manifestation of tetravalency by carbon atoms in organic substances. The theory also fulfilled its predictive function: on its basis, scientists predicted the existence of still unknown compounds, described their properties and discovered them. So, in 1862–1864. A. M. Butlerov examined propyl, butyl and amyl alcohols, determined the number of possible isomers and derived the formulas of these substances. Their existence was later experimentally proven, and some of the isomers were synthesized by Butlerov himself.
During the 20th century. the provisions of the theory of the chemical structure of chemical compounds were developed on the basis of new views that spread in science: the theory of atomic structure, the theory of chemical bonds, ideas about the mechanisms of chemical reactions. Currently, this theory is universal, that is, it is valid not only for organic substances, but also for inorganic ones.
First position. Atoms in molecules are combined in a specific order according to their valency. Carbon in all organic and most inorganic compounds is tetravalent.
Obviously, the last part of the first position of the theory can be easily explained by the fact that in compounds the carbon atoms are in an excited state:
Tetravalent carbon atoms can combine with each other to form different chains:

The order of connection of carbon atoms in molecules can be different and depends on the type of covalent chemical bond between carbon atoms - single or multiple (double and triple):

This position explains the phenomenon.
Substances that have the same composition, but different chemical or spatial structures, and therefore different properties, are called isomers.
Main types:
Structural isomerism, in which substances differ in the order of bonding of atoms in molecules: carbon skeleton

![]()
![]()
![]()

![]()
![]()
Third position. The properties of substances depend on the mutual influence of atoms in molecules.
For example, in acetic acid only one of the four hydrogen atoms reacts with an alkali. Based on this, it can be assumed that only one hydrogen atom is bonded to oxygen:
On the other hand, from the structural formula of acetic acid we can conclude that it contains one mobile hydrogen atom, that is, that it is monobasic.The main directions of development of the theory of the structure of chemical compounds and its significance.
During the time of A.M. Butlerov, organic chemistry was widely used
empirical (molecular) and structural formulas. The latter reflect the order of connection of atoms in a molecule according to their valence, which is indicated by dashes.
For ease of recording, abbreviated structural formulas are often used, in which dashes indicate only the bonds between carbon atoms or carbon and oxygen.
And fibers, products from which are used in technology, everyday life, medicine, and agriculture. The significance of the theory of chemical structure of A.M. Butlerov for organic chemistry can be compared with the significance of the Periodic Law and the Periodic Table of Chemical Elements of D.I. Mendeleev for inorganic chemistry. It is not for nothing that both theories have so much in common in the ways of their formation, directions of development and general scientific significance.
Theory of the structure of organic compounds: homology and isomerism (structural and spatial). Mutual influence of atoms in molecules
Theory of the chemical structure of organic compounds by A. M. Butlerov
Just as for inorganic chemistry the basis of development is the Periodic Law and the Periodic Table of Chemical Elements of D. I. Mendeleev, for organic chemistry the theory of the structure of organic compounds of A. M. Butlerov became fundamental.
The main postulate of Butlerov’s theory is the position about chemical structure of a substance, which refers to the order, the sequence of mutual connection of atoms into molecules, i.e. chemical bond.
Chemical structure refers to the order of combination of atoms of chemical elements in a molecule according to their valency.
This order can be displayed using structural formulas, in which the valencies of atoms are indicated by dashes: one dash corresponds to the unit of valence of an atom of a chemical element. For example, for the organic substance methane, which has the molecular formula $CH_4$, the structural formula looks like this:
The main provisions of the theory of A. M. Butlerov
- Atoms in molecules of organic substances are bonded to each other according to their valency. Carbon in organic compounds is always tetravalent, and its atoms are capable of combining with each other, forming various chains.
- The properties of substances are determined not only by their qualitative and quantitative composition, but also by the order of connection of atoms in the molecule, i.e., the chemical structure of the substance.
- The properties of organic compounds depend not only on the composition of the substance and the order of connection of atoms in its molecule, but also on the mutual influence of atoms and groups of atoms on each other.
The theory of the structure of organic compounds is a dynamic and developing doctrine. As knowledge about the nature of chemical bonds and the influence of the electronic structure of molecules of organic substances developed, they began to use, in addition to empirical And structural, electronic formulas. Such formulas indicate the direction of displacement of electron pairs in the molecule.
Quantum chemistry and the chemistry of the structure of organic compounds have confirmed the doctrine of the spatial direction of chemical bonds ( cis- And trans isomerism), studied the energy characteristics of mutual transitions in isomers, made it possible to judge the mutual influence of atoms in the molecules of various substances, and created the prerequisites for predicting the types of isomerism and the direction and mechanism of chemical reactions.
Organic substances have a number of features:
- All organic substances contain carbon and hydrogen, so when burned they form carbon dioxide and water.
- Organic substances are complex and can have a huge molecular weight (proteins, fats, carbohydrates).
- Organic substances can be arranged in rows of homologues similar in composition, structure and properties.
- For organic substances it is characteristic isomerism.
Isomerism and homology of organic substances
The properties of organic substances depend not only on their composition, but also on the order of connection of atoms in the molecule.

Isomerism- this is the phenomenon of the existence of different substances - isomers with the same qualitative and quantitative composition, i.e. with the same molecular formula.
There are two types of isomerism: structural And spatial (stereoisomerism). Structural isomers differ from each other in the order of bonding of atoms in the molecule; stereoisomers - the arrangement of atoms in space with the same order of bonds between them.
The following types of structural isomerism are distinguished: carbon skeleton isomerism, positional isomerism, isomerism of various classes of organic compounds (interclass isomerism).
Structural isomerism
Carbon skeleton isomerism is due to the different order of bonds between the carbon atoms forming the skeleton of the molecule. As has already been shown, the molecular formula $C_4H_(10)$ corresponds to two hydrocarbons: n-butane and isobutane. For the hydrocarbon $С_5Н_(12)$ three isomers are possible: pentane, isopentane and neopentane:
$CH_3-CH_2-(CH_2)↙(pentane)-CH_2-CH_3$

As the number of carbon atoms in a molecule increases, the number of isomers increases rapidly. For hydrocarbon $С_(10)Н_(22)$ there are already $75$, and for hydrocarbon $С_(20)Н_(44)$ - $366,319$.
Position isomerism due to the different position of the multiple bond, substituent, functional group with the same carbon skeleton of the molecule:
$CH_2=(CH-CH_2)↙(butene-1)-CH_3$ $CH_3-(CH=CH)↙(butene-2)-CH_3$
$(CH_3-CH_2-CH_2-OH)↙(n-propyl alcohol(propanol-1))$

Isomerism of various classes of organic compounds (interclass isomerism) is caused by different positions and combinations of atoms in molecules of substances that have the same molecular formula, but belong to different classes. Thus, the molecular formula $C_6H_(12)$ corresponds to the unsaturated hydrocarbon hexene-1 and the cyclic hydrocarbon cyclohexane:

The isomers are a hydrocarbon related to alkynes - butine-1 and a hydrocarbon with two double bonds in the butadiene-1,3 chain:
$CH≡C-(CH_2)↙(butyne-1)-CH_2$ $CH_2=(CH-CH)↙(butadiene-1,3)=CH_2$
Diethyl ether and butyl alcohol have the same molecular formula $C_4H_(10)O$:
$(CH_3CH_2OCH_2CH_3)↙(\text"diethyl ether")$ $(CH_3CH_2CH_2CH_2OH)↙(\text"n-butyl alcohol (1-butanol)")$
The structural isomers are aminoacetic acid and nitroethane, corresponding to the molecular formula $C_2H_5NO_2$:

Isomers of this type contain different functional groups and belong to different classes of substances. Therefore, they differ in physical and chemical properties much more than carbon skeleton isomers or positional isomers.
Spatial isomerism
Spatial isomerism is divided into two types: geometric and optical. Geometric isomerism is characteristic of compounds containing double bonds and cyclic compounds. Since free rotation of atoms around a double bond or in a ring is impossible, substituents can be located either on one side of the plane of the double bond or ring ( cis-position), or on opposite sides ( trance-position). Designations cis- And trance- usually referred to as a pair of identical substituents:

Geometric isomers differ in physical and chemical properties.
Optical isomerism occurs when the molecule is incompatible with its image in the mirror. This is possible when the carbon atom in the molecule has four different substituents. This atom is called asymmetrical. An example of such a molecule is the $α$-aminopropionic acid ($α$-alanine) molecule $CH_3CH(NH_2)COOH$.
The $α$-alanine molecule cannot coincide with its mirror image no matter how it moves. Such spatial isomers are called mirror, optical antipodes, or enantiomers. All physical and almost all chemical properties of such isomers are identical.
The study of optical isomerism is necessary when considering many reactions occurring in the body. Most of these reactions occur under the action of enzymes - biological catalysts. The molecules of these substances must fit the molecules of the compounds on which they act, like a key to a lock; therefore, the spatial structure, the relative arrangement of sections of the molecules and other spatial factors are of great importance for the course of these reactions. Such reactions are called stereoselective.
Most natural compounds are individual enantiomers, and their biological action differs sharply from the properties of their optical antipodes obtained in the laboratory. Such a difference in biological activity is of great importance, since it underlies the most important property of all living organisms - metabolism.
Homologous series is a series of substances arranged in increasing order of their relative molecular masses, similar in structure and chemical properties, where each member differs from the previous one by the homological difference $CH_2$. For example: $CH_4$ - methane, $C_2H_6$ - ethane, $C_3H_8$ - propane, $C_4H_(10)$ - butane, etc.
Types of bonds in molecules of organic substances. Hybridization of carbon atomic orbitals. Radical. Functional group.
Types of bonds in molecules of organic substances.
In organic compounds, carbon is always tetravalent. In the excited state, a pair of $2s^3$ electrons is broken in its atom and one of them moves to the p-orbital:

Such an atom has four unpaired electrons and can participate in the formation of four covalent bonds.
Based on the given electronic formula for the valence level of a carbon atom, one would expect that it contains one $s$-electron (spherical symmetric orbital) and three $p$-electrons having mutually perpendicular orbitals ($2р_х, 2р_у, 2p_z$- orbital). In reality, all four valence electrons of the carbon atom completely equivalent and the angles between their orbitals are equal to $109°28"$. In addition, calculations show that each of the four chemical bonds of carbon in a methane molecule ($CH_4$) is $25%$ $s-$ and $75%$ $p $-connection, i.e. mixing$s-$ and $p-$ states of electrons. This phenomenon is called hybridization, and mixed orbitals - hybrid.
A carbon atom in the $sp^3$-valence state has four orbitals, each of which contains one electron. In accordance with the theory of covalent bonds, it has the ability to form four covalent bonds with atoms of any monovalent elements ($CH_4, CHCl_3, CCl_4$) or with other carbon atoms. Such connections are called $σ$-connections. If a carbon atom has one $C-C$ bond, then it is called primary($Н_3С-СН_3$), if two - secondary($Н_3С-СН_2-СН_3$), if three - tertiary ( ), and if four - quaternary (
), and if four - quaternary ( ).
).
One of the characteristic features of carbon atoms is their ability to form chemical bonds by sharing only $p$ electrons. Such connections are called $π$-connections. $π$ bonds in molecules of organic compounds are formed only in the presence of $σ$ bonds between atoms. Thus, in the ethylene molecule $H_2C=CH_2$ the carbon atoms are connected by $σ-$ and one $π$ bond, in the acetylene molecule $HC=CH$ - by one $σ-$ and two $π$ bonds. Chemical bonds formed with the participation of $π$ bonds are called multiples(in an ethylene molecule - double, in an acetylene molecule - triple), and compounds with multiple bonds - unsaturated.
Phenomenon$sp^3$-, $sp^2$- And$sp$ - hybridization of the carbon atom.
When $π$ bonds are formed, the hybrid state of the atomic orbitals of the carbon atom changes. Since the formation of $π$-bonds occurs due to p-electrons, then in molecules with a double bond the electrons will have $sp^2$-hybridization (there was $sp^3$, but one p-electron goes to $π$- orbital), and with a triple one - $sp$-hybridization (two p-electrons went to the $π$-orbital). The nature of hybridization changes the direction of $σ$-bonds. If during $sp^3$-hybridization they form spatially branched structures ($a$), then during $sp^2$-hybridization all atoms lie in the same plane and the angles between $σ$-bonds are equal to $120°$(b) , and with $sp$-hybridization the molecule is linear (c):

In this case, the axes of the $π$-orbitals are perpendicular to the axis of the $σ$-bond.

Both $σ$- and $π$-bonds are covalent, which means they must be characterized by length, energy, spatial direction and polarity.
Characteristics of single and multiple bonds between C atoms.
Radical. Functional group.
One of the features of organic compounds is that in chemical reactions their molecules exchange not individual atoms, but groups of atoms. If this group of atoms consists only of carbon and hydrogen atoms, then it is called hydrocarbon radical, if it has atoms of other elements, then it is called functional group. So, for example, methyl ($СН_3$-) and ethyl ($С_2Н_5$-) are hydrocarbon radicals, and the hydroxy group (-$ОН$), aldehyde group (  ), nitro group (-$NO_2$), etc. are the functional groups of alcohols, aldehydes and nitrogen-containing compounds, respectively.
), nitro group (-$NO_2$), etc. are the functional groups of alcohols, aldehydes and nitrogen-containing compounds, respectively.
Typically, the functional group determines the chemical properties of an organic compound and is therefore the basis for their classification.
Lecture 15
Theory of the structure of organic substances. Main classes of organic compounds.
Organic chemistry – the science that studies organic matter. Otherwise it can be defined as chemistry of carbon compounds. The latter occupies a special place in the periodic table of D.I. Mendeleev for the variety of compounds, of which about 15 million are known, while the number of inorganic compounds is five hundred thousand. Organic substances have been known to mankind for a long time, such as sugar, vegetable and animal fats, dyes, fragrant and medicinal substances. Gradually, people learned by processing these substances to obtain a variety of valuable organic products: wine, vinegar, soap, etc. Advances in organic chemistry are based on achievements in the field of chemistry of protein substances, nucleic acids, vitamins, etc. Organic chemistry is of great importance for the development of medicine, since the vast majority of medicines are organic compounds not only of natural origin, but also obtained mainly through synthesis. The exceptional significance of the high molecular weight organic compounds (synthetic resins, plastics, fibers, synthetic rubbers, dyes, herbicides, insecticides, fungicides, defoliants...). Organic chemistry is of great importance for the production of food and industrial goods.
Modern organic chemistry has deeply penetrated into the chemical processes occurring during the storage and processing of food products: the processes of drying, rancidity and saponification of oils, fermentation, baking, fermentation, production of drinks, in the production of dairy products, etc. The discovery and study of enzymes and perfumes and cosmetics also played a major role.
One of the reasons for the wide variety of organic compounds is the uniqueness of their structure, which is manifested in the formation of covalent bonds and chains by carbon atoms, varying in type and length. Moreover, the number of bonded carbon atoms in them can reach tens of thousands, and the configuration of carbon chains can be linear or cyclic. In addition to carbon atoms, the chains may contain oxygen, nitrogen, sulfur, phosphorus, arsenic, silicon, tin, lead, titanium, iron, etc.
The manifestation of these properties by carbon is due to several reasons. It was confirmed that the energies of the C–C and C–O bonds are comparable. Carbon has the ability to form three types of orbital hybridization: four sp 3 - hybrid orbitals, their orientation in space is tetrahedral and corresponds to simple covalent bonds; three hybrid sp 2 orbitals located in the same plane, in combination with a non-hybrid orbital, form double multiples connections (─С = С─); also with the help of sp - hybrid orbitals of linear orientation and non-hybrid orbitals between carbon atoms arise triple multiples bonds (─ C ≡ C ─). Moreover, carbon atoms form these types of bonds not only with each other, but also with other elements. Thus, the modern theory of the structure of matter explains not only a significant number of organic compounds, but also the influence of their chemical structure on their properties.
It also fully confirms the basics theories of chemical structure, developed by the great Russian scientist A.M. Butlerov. ITS main provisions:
1) in organic molecules, atoms are connected to each other in a certain order according to their valency, which determines the structure of the molecules;
2) the properties of organic compounds depend on the nature and number of atoms included in their composition, as well as on the chemical structure of the molecules;
3) each chemical formula corresponds to a certain number of possible isomer structures;
4) each organic compound has one formula and has certain properties;
5) in molecules there is a mutual influence of atoms on each other.
Classes of organic compounds
According to the theory, organic compounds are divided into two series - acyclic and cyclic compounds.
1. Acyclic compounds.(alkanes, alkenes) contain an open, unclosed carbon chain - straight or branched:
N N N N N N N
│ │ │ │ │ │ │
N─ S─S─S─S─ N H─S─S─S─N
│ │ │ │ │ │ │
N N N N N │ N
Normal butane isobutane (methylpropane)
2. a) Alicyclic compounds– compounds that have closed (cyclic) carbon chains in their molecules:
cyclobutane cyclohexane
b) Aromatic compounds, in the molecules of which there is a benzene skeleton - a six-membered ring with alternating single and double bonds (arenes):

c) Heterocyclic compounds– cyclic compounds containing, in addition to carbon atoms, nitrogen, sulfur, oxygen, phosphorus and some trace elements, which are called heteroatoms.

furan pyrrole pyridine
In each row, organic substances are distributed into classes - hydrocarbons, alcohols, aldehydes, ketones, acids, esters in accordance with the nature of the functional groups of their molecules.
There is also a classification according to the degree of saturation and functional groups. According to the degree of saturation they are distinguished:
1. Extremely saturated– the carbon skeleton contains only single bonds.
─С─С─С─
2. Unsaturated unsaturated– in the carbon skeleton there are multiple (=, ≡) bonds.
─С=С─ ─С≡С─
3. Aromatic– unsaturated cycles with ring conjugation (4n + 2) π-electrons.
By functional groups
1. Alcohols R-CH 2 OH
2. Phenols 
3. Aldehydes R─COH Ketones R─C─R
4. Carboxylic acids R─COOH O
5. Esters R─COOR 1
By the first half of the 19th century, an enormous amount of factual material had been accumulated in organic chemistry, the further study of which was hampered by the lack of any systematizing basis. Starting from the 20s of the 19th century, successive theories began to appear, claiming to provide a generalized description of the structure of organic compounds. One of them was the theory of types, developed in the 1960s by the French scientist C. Gerard. According to this theory, all organic compounds were considered as derivatives of the simplest inorganic substances, taken as types.Sh. Gerard
Shortly before the appearance of the theory of the structure of A.M. Butlerov, the German chemist F.A. Kekule (1857) developed the theory of valency in relation to organic compounds, which established such facts as the tetravalency of the carbon atom and its ability to form carbon chains due to combination with carbon atoms.A. M. Butlerova F.A. Kekule

Theoretical developments of the pre-Butler period made a certain contribution to the knowledge of the structure of organic compounds. But none of the early theories was universal. And only A.M. Butlerov managed to create such a logically complete theory of structure, which to this day serves as the scientific basis of organic chemistry. Theory of the structure of A.M. Butlerov is based on a materialistic approach to a real molecule and proceeds from the possibility of knowing its structure experimentally. A.M. Butlerov attached fundamental importance to chemical reactions when establishing the structure of substances. Theory of the structure of A.M. Butlerova not only explained already known facts, her scientific significance lay in predicting the existence of new organic compounds. A.M. Butlerov A.M. Butlerova A.M. Butlerov A.M. Butlerov


Isomers are substances that have the same molecular formula, but different chemical structures, and therefore have different properties. Isomerism received a true explanation only in the second half of the 19th century on the basis of the theory of chemical structure by A.M. Butlerov (structural isomerism) and the stereochemical teachings of Ya. G. Van't Hoff (spatial isomerism). Ya. G. van't Hoff

FormulaName Number of isomers CH 4 methane1 C4H6C4H6 ethane1 C3H8C3H8 propane1 C 4 H 10 butane2 C 5 H 12 pentane3 C 6 H 14 hexane5 C 7 H 16 heptane9 C 8 H 18 octane18 C 9 H 20 nonane35 C 10 H 22 decane75 C 11 H 2 4 undecane159 C 12 H 26 dodecane355 C 13 H 28 tridecane802 C 14 H 30 tetradecane1 858 C 15 H 32 pentadecane4 347 C 20 H 42 eicosane C 25 H 52 pentacosane C 30 H 62 triacontane C 40 H 82 tetracontane

Structural isomers are those that correspond to different structural formulas of organic compounds (with different orders of atoms). Spatial isomers have identical substituents on each carbon atom and differ only in their relative location in space.

Spatial isomers (stereoisomers). Stereoisomers can be divided into two types: geometric isomers and optical isomers. Geometric isomerism is characteristic of compounds containing a double bond or ring. In such molecules it is often possible to draw a conventional plane in such a way that the substituents on different carbon atoms can be on the same side (cis-) or on opposite sides (trans-) of this plane. If a change in the orientation of these substituents relative to the plane is possible only due to the breaking of one of the chemical bonds, then they speak of the presence of geometric isomers. Geometric isomers differ in their physical and chemical properties.





A new method for obtaining optical isomers of organic molecules has been discovered. When Alice found herself in her own, but “mirror” room, she was surprised: the room seemed similar, but still completely different. Mirror isomers of chemical molecules differ in the same way: they look similar, but behave differently. A critical area of organic chemistry is the separation and synthesis of these mirror variants. (Illustration by John Tenniel for Lewis Carroll's book "Alice Through the Looking Glass")


American scientists have learned to obtain optical isomers of aldehyde-based compounds, finally carrying out an important reaction that chemists have been working on for many years. In the experiment, they combined two catalysts operating on different principles. As a result of the combined action of these catalysts, two active organic molecules are formed, which combine to form the desired substance. Using this reaction as an example, the possibility of synthesizing a whole class of biologically important organic compounds is demonstrated.

At least 130 organic synthesis reactions are now known in which more or less pure chiral isomers are obtained. If the catalyst itself has chiral properties, then an optically active product will be obtained from an optically inactive substrate. This rule was derived at the beginning of the 20th century and remains basic today. The principle of selective action of a catalyst in relation to optical isomers is similar to a handshake: it is “convenient” for the catalyst to bind to only one of the chiral isomers, and therefore only one of the reactions is preferentially catalyzed. By the way, the term “chiral” comes from the Greek chéir hand.