Vadim Valerievich Korpachev healing fauna. Proteins, their structure and functions
Chapter III. PROTEINS
§ 6. AMINO ACIDS AS STRUCTURAL ELEMENTS OF PROTEINS
Natural amino acids
Amino acids in living organisms are found mainly in proteins. Proteins are composed primarily of twenty standard amino acids. They are a-amino acids and differ from each other in the structure of side groups (radicals), designated by the letter R:
The variety of side radicals of amino acids plays a key role in the formation of the spatial structure of proteins and in the functioning of the active center of enzymes.
The structure of standard amino acids is given at the end of the paragraph in Table 3. Natural amino acids have trivial names, which are inconvenient to use when writing the structure of proteins. Therefore, three-letter and one-letter designations have been introduced for them, which are also presented in Table 3.
Spatial isomerism
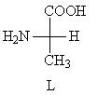
In all amino acids, with the exception of glycine, the a-carbon atom is chiral, i.e. They are characterized by optical isomerism. In table The 3 chiral carbon atom is indicated by an asterisk. For example, for alanine, the Fischer projections of both isomers look like this:
To designate them, as for carbohydrates, D, L-nomenclature is used. Proteins contain only L-amino acids.
L- and D-isomers can be mutually converted into each other. This process is called racemization.
Interesting to know! In the white of teeth - dentin -L-asparticthe acid spontaneously racemizes at human body temperature at a rate of 0.10% per year. During the period of tooth formation, dentin contains onlyL-aspartic acid, in an adult, as a result of racemization, it is formedD-aspartic acid. The older the person, the higher the D-isomer content. By determining the ratio of D- and L-isomers, the age can be determined quite accurately. Thus, the inhabitants of the mountain villages of Ecuador were exposed for attributing too much age to themselves.
Chemical properties
Amino acids contain amino and carboxyl groups. Because of this, they exhibit amphoteric properties, that is, the properties of both acids and bases.
When an amino acid, such as glycine, is dissolved in water, its carboxyl group dissociates to form a hydrogen ion. Next, the hydrogen ion attaches due to the lone pair of electrons at the nitrogen atom to the amino group. An ion is formed in which positive and negative charges are simultaneously present, the so-called zwitterion:
This form of the amino acid is predominant in a neutral solution. In an acidic environment, an amino acid attaches a hydrogen ion to form a cation:
In an alkaline environment an anion is formed:
Thus, depending on the pH of the environment, an amino acid can be positively charged, negatively charged and electrically neutral (with equal positive and negative charges). The pH value of a solution at which the total charge of an amino acid is zero is called isoelectric point of this amino acid. For many amino acids, the isoelectric point lies near pH 6. For example, the isoelectric points of glycine and alanine have values of 5.97 and 6.02, respectively.
Two amino acids can react with each other, causing a water molecule to be split off and forming a product called dipeptide:
The bond connecting two amino acids is called peptide bond. Using the letter designations of amino acids, the formation of a dipeptide can be schematically represented as follows:
Similarly formed tripeptides, tetrapeptides etc.:
H 2 N – lys – ala – gly – COOH – tripeptide
H 2 N – trp – gis – ala – ala – COOH – tetrapeptide
H 2 N – tyr – lys – gly – ala – leu – gly – trp – COOH – heptapeptide
Peptides consisting of a small number of amino acid residues have the general name oligopeptides.
Interesting to know! Many oligopeptides have high biological activity. These include a number of hormones, for example, oxytocin (nanopeptide) stimulates uterine contractions, bradykinin (nanopeptide) suppresses inflammatory processes in tissues. The antibiotic gramicidin C (cyclic decapeptide) disrupts the regulation of ion permeability in bacterial membranes and thereby kills them. Mushroom poisons amanitins (octapeptides), by blocking protein synthesis, can cause severe poisoning in humans. Aspartame is widely known - the methyl ester of aspartyl phenylalanine. Aspartame has a sweet taste and is used to add a sweet taste to various foods and drinks.
Classification of amino acids
There are several approaches to the classification of amino acids, but the most preferred is the classification based on the structure of their radicals. There are four classes of amino acids containing the following types of radicals; 1) non-polar ( or hydrophobic); 2) polar uncharged; 3) negatively charged and 4) positively charged:
Non-polar (hydrophobic) include amino acids with non-polar aliphatic (alanine, valine, leucine, isoleucine) or aromatic (phenylalanine and tryptophan) R-groups and one sulfur-containing amino acid - methionine.
Polar uncharged amino acids, compared to non-polar ones, are better soluble in water and are more hydrophilic, since their functional groups form hydrogen bonds with water molecules. These include amino acids containing a polar HO group (serine, threonine and tyrosine), an HS group (cysteine), an amide group (glutamine, asparagine) and glycine (the glycine R group, represented by one hydrogen atom, is too small to compensate strong polarity of the a-amino group and the a-carboxyl group).
Aspartic and glutamic acids are negatively charged amino acids. They contain two carboxyl and one amino groups, so in the ionized state their molecules will have a total negative charge:
Positively charged amino acids include lysine, histidine and arginine; in ionized form they have a total positive charge:
Depending on the nature of the radicals, natural amino acids are also divided into neutral, sour And basic. Neutral includes non-polar and polar uncharged, acidic - negatively charged, basic - positively charged.
Ten of the 20 amino acids that make up proteins can be synthesized in the human body. The rest must be contained in our food. These include arginine, valine, isoleucine, leucine, lysine, methionine, threonine, tryptophan, phenylalanine and histidine. These amino acids are called irreplaceable. Essential amino acids are often included in food supplements and used as medicines.
Interesting to know! The balance of human nutrition in amino acids plays an extremely important role. If there is a lack of essential amino acids in food, the body self-destructs. In this case, the brain is primarily affected, which leads to various diseases of the central nervous system and mental disorders. A young growing organism is especially vulnerable. For example, when the synthesis of tyrosine from phenylalanine is disrupted, children develop a severe disease, finylpyruvic oligophrenia, which causes severe mental retardation or the death of the child.
Table 3
Standard amino acids
|
Amino acid (trivial name) |
Legend |
Structural formula |
||
|
Latin |
||||
|
three-letter |
single-letter |
|||
|
NON-POLAR (HYDROPHOBIC) |
||||
|
Isoleucine |
|
|||
|
Phenylalanine |
||||
|
Tryptophan |
|
|||
|
Methionine |
||||
|
POLAR UNCHARGED |
||||
|
Asparagine |
|
|||
|
Glutamine | ||||
Splenin has found widespread use in healthcare practice. This spleen preparation was obtained in 1945 at the Laboratory of Experimental Endocrinology (Institute of Experimental Biology and Pathology named after A. A. Bogomolets) by Academician of the Academy of Sciences of the Ukrainian SSR V. P. Komissarenko. The chemical nature of splenin has been studied in some detail. The preparation contains a large number of amino acids, a peptide containing 13 amino acids, many fatty acids, as well as lipids, trace elements and vitamins. The active principle of splenin has not yet been isolated.
Experiments on various animal species showed a pronounced detoxifying effect of the drug.
A test of the effect of splenin in toxicosis in early pregnancy, carried out in various institutions in our country, showed that it is highly effective in the treatment of this pathology. In addition, using splenin in the treatment of complications in patients after radiotherapy, doctors noticed that after 3–4 injections of the drug the general condition of a person improves significantly: nausea and vomiting, headaches stop, appetite appears, and sleep normalizes. Due to its pronounced detoxifying properties, the drug has a pronounced therapeutic effect in the treatment of various forms of hepatitis and functional liver disorders, thyrotoxicosis, parathyroid insufficiency, schizophrenia and diabetes.
Researchers have discovered another ability of the drug - to suppress the manifestation of allergic reactions. Splenin had a pronounced therapeutic effect in the treatment of allergic rhinitis, urticaria and allergic dermatitis.
Many of the effects of splenin can be explained by its membranotropic properties, i.e., the ability to stabilize the cell membrane. Thus, red blood cells treated with this drug are less sensitive to hypotonic shock. The mechanism of many of the effects of splenin has not yet been sufficiently studied. The chemical nature of the biologically active factors included in its composition has not been clarified. Study of the drug continues.
Currently, only two peptides have been isolated from the spleen, the structure of which has been established: 1. Tuftsin, the biosynthesis of which occurs in the spleen in the form of leukokinin, and the final structure is formed on the surface of leukocyte membranes. Currently, tuftsin has been synthesized, and its biologically active analogues have also been obtained. 2. A factor resembling thymopoietin in its structure and called splenin. It, like thymopoietin, consists of 49 amino acids and has an active site of five
Tir-Liz-Pro-Arg
Taftsin
amino acids, which was named splenopentin. Splenopentin differs from thymopentin in one amino acid.
Arg-Lys-Asp-Val-Tir
Timopentin
Arg-Liz-Glu-Val-Tyr
Splenopentine
The biological effects of splenopentin and thymopentin are significantly different.
The study of humoral factors of the spleen is carried out at the Kiev Research Institute of Endocrinology and Metabolism. In recent years, a number of new important data have been obtained here, which have made it possible to significantly expand our understanding of the physiology and pathology of the functions of the spleen, and the significance of those phenomena that arise when it is disrupted. However, many mysteries of this organ remain unsolved.
Paradoxes of the animal world
When studying biologically active substances of various natures and different origins, it becomes obvious that they are conventionally divided into mediators that provide intercellular connections, hormones that transmit signals over longer distances, pheromones that are means of communication between organisms, and toxins that serve animals for protection.
Analysis of the structure of biological regulators shows that the same compound can perform different roles in different species of the animal kingdom. Luliberin acts as a hormone in the hypothalamic-pituitary system, while the same peptide in the sympathetic ganglion of the frog acts as a neurotransmitter. The mating pheromone in yeast α-factor binds to the receptors of the mammalian pituitary gland and, when acting on gonadotropes in tissue culture, causes the secretion of luteinizing hormone. The study of its chemical composition showed that it has extensive amino acid sequence homology with luliberin.
Structural homology plays an important role in the interaction of a biostimulant with a receptor, while the physiological response is determined by the functional system on which it acts.
In 1931, von Euler and Gaddum discovered a substance in extracts of the brain and intestines of animals that, when administered to an anesthetized rabbit, caused a decrease in blood pressure and increased contraction of the isolated intestine. It was called "substance P". It was later found that it is a neurotransmitter of sensitive neurons and its content in the dorsal (sensitive) roots of the spinal cord is twice as high as the concentration in the anterior roots. The structure of the substance was determined 40 years later, and it turned out that it is similar to the structure of peptides such as physalemin, isolated from the skin of a South African frog, and eledosin, found in the salivary glands of octopuses.
Arg-Pro-Lys-Pro-Gly-Gly-Fen-Gly-Leu-Met-NH 2
Substance P
Piroglu-Ala-Asp-Pro-Asp-Lys-Fep-Tri-Gly-Leu-Met-NH 2
Physalemin
Piroglu-Pro-Ser-Liz-Asp-Ala-Fen-Iley-Gly-Gly-Ley-Met-NH 2
Eledozin
These three substances have a similar structure, including homologous peptide regions, while they are obtained from different sources and perform different functions.
Another example is the peptide bombesin, which was isolated from the skin of the European frog Bombina bombina and then found in P cells of the gastric and duodenal mucosa of mammals. Bombesin functions as a releasing factor in the release of gastrin and cholecystokinin. In this regard, it causes stimulation of the stomach and pancreas, contracts the gallbladder and increases bowel movement. Using immunological research methods, it was found that the nerve cells of the cerebral cortex, hypothalamus, pituitary gland, pineal gland and cerebellum, in addition to the usual hormones of the digestive organs, also contain bombesin. It has no equal among known substances in its ability to affect thermoregulation. When it is introduced into the hypothalamic structure of the rat's brain at 4°C, a decrease in body temperature occurs - it turns out to be several degrees lower than usual in the rat. At 36° the body temperature increased. This peptide was effective only when injected into the hypothalamus, where the thermoregulatory center is located. This property is probably associated with its participation in the hibernation of some animals. The injection of bombesin into the ventricles of a rat's brain caused a change in behavior and a decrease in pain sensitivity. In addition, it increases blood glucose, increases glucagon concentrations, decreases insulin levels and inhibits food intake in hungry rats. This is the only peptide that regulates the feeling of fullness, since it does not affect the frequency of food intake, but only the amount eaten. The entry of bombesin into the ventricles of the brain prevented the occurrence of stomach ulcers during stress. At the same time, the secretion of hydrochloric acid decreased and the excretion of mucus increased. Bombesin also stimulates the secretion of somatotropic and lactotropic hormones. Its properties suggest that it is a neurotransmitter in nerve structures.
In the foreign journal “Biochem. J." (1981. T. 197, No. 3) a report was published that a substance similar to the polypeptide of the mammalian pancreas was isolated from the heads of the carrion fly Calliphora vomitoria, and in another foreign journal (Insect. Biochem. 1977. T. 7. No. 5 – 6) protein fractions isolated from the beetles Adalia bipunctata, butterflies Galleria mellonella and bees are described, which in their properties are close to the somatotropic hormone of bovine blood serum.
Special photo 218 (=^_^ =)
The basis is taken from old problems - 113 pieces. I simply won’t print those that were removed from the new list. Although I simply don’t have some. The answers here are of course not perfect, but still, at least you can take something from here.
Q: Chemical bonds can occur between amino acid radicals in a peptide chain. Select pairs of amino acids that can form them: a) val-isoles; b) cis-cis; c) glu-lyse; d) fen-asp. Justify your answer.
A: a) form a bond – a hydrophobic bond, because both amino acids are hydrophobic
b) form a bond - a disulfide bond due to SH
c) form a bond – an ionic bond, because acidic and basic amino acids
d) do not form bonds
2. Q: Write the formula of the tripeptide: lys-arg-his. In what environment is his IET located? Justify your answer.
A: IEP is the pH value at which the protein charge approaches zero. All amino acids in this tripeptide are basic. This means IET in an alkaline environment.
3: Q: Write the formula of the tripeptide: glu-asp-ala. Determine its charge in a neutral environment. How does the charge change in an acidic environment? Solubility? Why?
A: Glu and asp are acidic amino acids, ala is hydrophobic. At pH = 7, the charge of the tripeptide is negative. In an acidic environment (with the addition of H + protons), the charge will decrease, i.e. tend to IET. As the charge decreases, solubility also decreases.
4. Q: Tripeptide: val-ley-ala. Check its ability to dissolve in water. Why?
A: The tripeptide is formed by hydrophobic amino acids, between the radicals of which hydrophobic bonds are formed. Therefore, it does not dissolve in water.
5. B : Two patients are sick with dysentery. One has a protein coefficient of 0.9, the other 1.9. What is the doctor's tactics in both cases?
A: Dysentery is an infectious disease. Caused by the introduction of a microorganism into the macroorganism.
BC is the ratio of albumin to globulin in the blood serum. BC= [A]/[G]=1.5 – 2.3. When a microorganism is introduced, humoral immunity is triggered, which causes the production of gamma globulins. With an increase in globulins in the blood, the BC value decreases, which means the body is fighting the infection - as in the first patient. In the second case, BC = 1.9, i.e. gamma globulins are not produced, which means the body does not fight the microorganism. Consequently, the condition is worse in the second patient. In the first case, the doctor prescribes antibiotics that act on the membrane of the microbe, penetrate into the cell, and act on the DNA of the microorganism, thereby preventing it from multiplying. In the second case, immunostimulants are prescribed to adults, and ready-made antibodies are administered to children and the elderly. Also in the 1st and 2nd cases, water-salt solutions are given to prevent dehydration.
6. Q: Explain the mechanism of formation of kefir from milk. Why are kefir proteins better absorbed by a child’s body?
A: Kefir is a product of fermented milk fermentation, as a result of the work of microorganisms whose food products are carbohydrates. The end product of this process is lactic acid. In milk, lactose undergoes fermentation to form lactic acid. This changes the pH from neutral to acidic. The vast majority of proteins are acidic, including milk proteins. When the pH changes, the charge of the proteins also changes - it decreases, and solubility also decreases. Lactic acid acts on the chemical bonds between the amino acid radicals of milk proteins, breaks these bonds - that is, denaturation occurs) the proteins unfold). In kefir, proteins do not precipitate, but remain on the surface. Children use kefir proteins, because they are in expanded form and are better absorbed - because peptide bonds are available.
7. Q: Which blood plasma proteins, albumins or globulins, move faster during electrophoresis? Why?
A: Electrophoresis is the movement of particles of a solution placed in an electric field. The speed of particle movement is directly proportional to the charge, and inversely proportional to the mass of the particles. Albumins and globulins are acidic proteins, which means they have a “-” charge, but albumins have a higher charge. By weight: albumins are smaller than globulins. This means that according to the 2nd criteria, it is clear that albumin moves faster during electrophoresis.
8. Q: Where in medicine is the ability of proteins to denature used?
A: Three areas of medicine
A) PREVENTION: quartz treatment, treatment of the surgical field, treatment of the skin before the injection, sterilization.
B) TREATMENT: chemotherapy - destruction of the tumor, stopping bleeding - coagulation (clotting)
C) DIAGNOSTICS: precipitation of proteins to determine their quantity, precipitation to determine the quantity of other substances in biological fluids.
9. Q: Which is more soluble in water, nucleic acids or nucleoproteins? Why?
As the charge decreases, solubility decreases. This means that NCs dissolve better in water.
10. Q: What are the dangers of excessive attempts to tan?
A: When trying to tan, the synthesis of melanin in the body increases. This means that the effect of UVR is not very preferable. If the body does not produce enough melanin, then UV penetrates into the deeper layers of the skin. This is how it acts on skin cells. Namely, on their membranes. Cell membranes are based on a bilipid layer consisting of PL and IVH. And IVZhK have the following type of structure: -С-С-С-.. i.e. chain of carbon atoms. And the UV ray is a bunch of energy that breaks this chain according to the homolytic type of bond disintegration, i.e. Free radicals with an unpaired electron are formed. It is these radicals that trigger LPO. During this process, the cell membrane is damaged, and the UV ray penetrates inside. And inside the cell there are large quantities of proteins and NK, which are polymers. When exposed to UV radiation, denaturation occurs. Consequences: burns, tumors. Also, with excessive attempts to tan, excessive synthesis of vitamin D is provoked, which can lead to hypervitaminosis, the consequences of which are an increase in the concentration of Ca2+ in the blood, and it can be deposited in tissues and organs, disrupting their activity.
11. : Q: Pepsin has an IET of 1. What amino acids predominate in its molecule?
A: Acidic amino acids.
12: Q: Have you ever observed the work of enzymes? Give examples.
A: Carbohydrates: fermentation - lactic acid, alcoholic, citric acid, acetic acid, etc. (yogurt, kefir, kvass, sauerkraut, sour dough)
Lipids: rancidity of butter
Proteins: rotting (rotten eggs, spoiled food, moldy cheese)
13. Q: Write the reaction for the decarboxylation of glutamate. Name the class of enzyme. The role of the product.
ABOUT: 
The product is gamma-aminobutyric acid, an inhibitory neurotransmitter. The enzyme is GLUTAMATE DECARBOXYLASE. Enzyme class - lyase (breaks –C-C bond)
14. Q: Specify the enzyme classaldolases glycolysis. Justify your answer
A: The enzyme class is lyase. The -S-S- connection breaks. With the help of this enzyme, fructose-1,6-bisphosphate is converted into GA-3-P and DHAP.

not in new tasks!
Q: What is more dangerous than a burn from an acid or an alkali (with equal ionic strength)? Explain your answer.
A: Burns from alkali are more dangerous. Both acid and alkali, when in contact with the skin, cause denaturation of tissue proteins. But the acid has the property of being hygroscopic. This property causes the capture of water from the fabric, i.e. when it comes into contact with the skin, the acid seems to draw out all the water from the fabric and a dry crust remains. It is an obstacle to further penetration of acid into the deeper layers of the skin. Alkali does not have this property and therefore can cause denaturation of proteins in the deeper layers of the skin.
Q: For what purpose are operating rooms quartzed? The mechanism of the phenomenon.
A: Quartzization is the effect of ultraviolet radiation on any surface. Quartz treatment is used to neutralize microorganisms. This is based on the denaturation of the proteins of these microorganisms, and first of all, the hydrophobic interactions of amino acid radicals in peptides are broken, which “opens” the peptide bonds of the primary structure of the protein.
Q: First aid for poisoning with heavy metal salts. Explain your answer
A: The main task is to prevent the absorption of these salts. To do this, a solution of natural proteins is injected inside. Most often it is milk. Milk proteins bind to salts, which causes their denaturation and they are deposited on the mucous membrane, reducing the access of salts to the mucous membrane. After this, rinsing is done.
Q: How much glucose is normal in urine and why?
A: Normally there is no glucose in the urine, because it is completely reabsorbed by the renal tubules. There is glucose in primary urine, but then it is completely reabsorbed, and reabsorption occurs through active transport, i.e. with energy consumption. With chronic hyperglycemia (9-10 mmol/l), the percentage of reabsorption of this compound decreases, because lack of energy and glucosuria may develop.
Q: Name the enzyme involved in the synthesis and breakdown of glycogen. Reaction formulas.
ABOUT: 
The enzyme is PHOSPHOHEXOMUTASE.
21.Q: What are the dangers of early and excessive consumption of easily digestible carbohydrates in children? Why?
A: Easily digestible carbohydrates can be given to children from 5 years of age. It is not possible before this date, because... is fraught with:
a) diathesis,
b) caries,
c) hyperglycemia is observed in the blood - which means the production of insulin, which leads to lipogenesis in lipocytes - as a consequence obesity, in the liver lipogenesis - fatty infiltration (degeneration) of the liver,
d) type II diabetes may develop,
e) cholesterol synthesis increases – atherosclerosis,
f) increased intake of cholesterol into the liver, excreted in bile, if its content in bile is exceeded - precipitation, gallstones.
Q: Difference in glycogen utilization in muscles and liver.

23.: Why does the block of glucose-6-phosphatase stimulate glycogen synthesis? An example of pathology.
A: Glucose-6-phosphatase deficiency is the basis of Gierke's disease, or glycogenosis type 1. Deficiency of this enzyme results in the inability to convert glucose-6-phosphate into glucose, which is accompanied by the accumulation of glycogen in the liver and kidneys. The disease is inherited in an autosomal recessive manner.
The intake of glucose into the body from food, in principle, makes it possible to maintain a normal level of glucose in the blood, but for this to happen, the intake of food containing glucose must be almost continuous. In real conditions of existence, i.e. in the absence of a continuous supply of glucose, in a healthy body the latter is deposited in the form of glycogen, which, if necessary, is used in its polymerization.
The primary disorder in Gierke's disease occurs at the genetic level. It consists of a complete or almost complete inability of cells to produce glucose-6-phosphatase, which ensures the cleavage of free glucose from glucose-6-phosphate. As a result, glycogenolysis is interrupted at the level of glucose-6-phosphate and does not go further. Dephosphorylation with the participation of glucose-6-phosphatase is a key reaction not only of glycogenolysis, but also of gluconeogenesis, which, therefore, in Gierke's disease is also interrupted at the level of glucose-6-phosphate. The occurrence of sustained hypoglycemia, which in real conditions is inevitable due to the lack of glucose entering the blood as the final product of glycogenolysis and gluconeogenesis, in turn leads to a constant increased secretion of glucagon as a stimulator of glycogenolysis. Glucagon, however, when this process is interrupted, can only continuously stimulate its initial stages without benefit to the body.
13.. Due to what bonds can a copolymer be formed from the two peptides below?
A) ala-met-arg-cis-ala-gli-ser-gli-cis-tre;
b) lys-glu-arg-cis-arg-gly-tre-ser-lys-tre-glu-ser.
14. How, using the biuret method for determining protein and ammonium sulfate, to establish the ratio between albumins and globulins in blood serum?
15. The ratio of the amount of albumin to the amount of globulin in the patient’s blood serum is 1.5. Calculate the globulin content if the albumin concentration is 5.0 g%.
16. Name the two main configurations of a protein molecule and indicate the differences between them.
17. At what level of spatial organization are globular and fibrillar proteins distinguished?
18. Name the most important groups of basic proteins.
19. Why do protamines and histones differ in their basic character?
20. Why do protamines and histones coagulate under high heat only in a highly alkaline environment?
LESSON 3 “Chemistry of complex proteins. Determination of components of phospho- and nucleoproteins"
Purpose of the lesson : become familiar with the classification and structure of complex proteins, especially nucleoproteins, which play a leading role in the storage and transmission of genetic information (DNA and RNA), as well as the most important chromoproteins (hemoglobin).
The student should know:
1. Classes of complex proteins, the principle of their division into classes, the principle of nomenclature
2. The chemical nature of prosthetic groups of complex proteins.
3. Components of the prosthetic group of nucleoproteins and chromoproteins (in particular, hemoglobin).
4. Spatial organization of nucleic acids.
5. Differences in the composition and structure of RNA and DNA
6.Functions of DNA and RNA, types of RNA, their localization.
7. Prosthetic group of hemoglobin, its components, the role of iron in the composition of heme.
8. Factors whose impact can cause changes in DNA structure with informational consequences.
The student must be able to:
1. Construct (schematically) a complementary chain to a section of a given fragment of one of the DNA chains.
2. Determine, based on the results of a qualitative analysis of nucleic acid hydrolyzate, whether DNA or RNA was hydrolyzed
3. Distinguish between the types of hemoglobin and use the designations adopted for them (oxyhemoglobin, reduced hemoglobin, carboxyhemoglobin, etc.
4. Find errors in segments of supposedly complementary DNA strands presented for evaluation
The student must get an idea: about the predominant localization of complex proteins in the human body, their biological significance, about the threats that mutagenic effects pose to the existence of species.
Classroom work
Laboratory work (Determination of phospho-
And nucleoproteins)
1. Isolation of casein from milk. Casein (one of the phosphoproteins) is contained in milk in the form of a soluble calcium salt, which decomposes when acidified, and casein precipitates. Excess acid interferes with precipitation, since at pH values below 4.7 (the isoelectric point of casein), the protein molecules are recharged and casein goes back into solution.
Progress. To 2 ml of milk add an equal volume of distilled water and 2 drops of 10% acetic acid. Collect the casein that falls out in the form of flakes on a filter and rinse with water.
Hydrolysis of nucleoproteins
Progress. Place 1 g of yeast in a round-bottomed flask, add 20 ml of a 10% sulfuric acid solution and the same amount of distilled water. Close the flask with a reflux stopper and boil under pressure for 1.5 hours at low heat. Cool the liquid, add distilled water to the original volume, and filter. Use the filtrate for the following qualitative reactions:
a) biuret reaction(for detection of polypeptides). To 5 drops of the resulting hydrolyzate add 10 drops of a 10% solution of sodium hydroxide and 1 drop of a 1% solution of copper sulfate. The liquid turns pink;
b) silver test(to detect purine bases). Add 5 drops of a 2% ammonia solution of silver nitrate to 5 drops of hydrolyzate. After 3-5 minutes, a small brown precipitate of silver compounds of purine bases precipitates;
c) qualitative Molisch reaction(to detect the pentose group). To 10 drops of hydrolyzate, add 2 - 3 drops of a 1% solution of thymol in ethanol, mix and lower an equal volume of concentrated sulfuric acid along the wall - a distinct red ring;
d) molybdenum sample(for phosphoric acid detection). Add 5 drops of molybdenum reagent to 5 drops of hydrolyzate and boil for several minutes. A lemon-yellow color appears, and upon cooling, a yellow crystalline precipitate of a complex compound of ammonium phosphomolybdate appears.
Give reasoned answers to the tasks suggested below:
1. What structural components make up DNA? In what order are they connected to each other?
2. Construct a complementary chain to the site. the DNA fragment shown below (- A - G - G - C - T- G-T) so that the resulting chain is an RNA fragment:
3. Construct a complementary chain to a section of one of the DNA chains presented below:
-A - G - G - C - T -
: - : - : - : - :
-? - ? - ? - ? - ? -
4.Find errors in the DNA fragment below:
-T - U - A - U - C - T - T - G-
: -: - : - : : : : :
A - A - T - A - G - A - A - U-
5. The oligonucleotide was hydrolyzed in two ways. In the first case, mononucleotides were determined in the hydrolyzate A, G, C and T(the latter is found in the hydrolyzate in an amount 2 times higher than the others), as well as dinucleotides G - A, A - T And T - T. In the second case, along with free nucleotides, a dinucleotide was found G - C.
Determine the nucleotide sequence in the original product?
6. The test solution exhibits a positive biuret reaction and forms a precipitate upon boiling and the addition of concentrated mineral acids, as well as sulfosalicylic acid.
Draw up a research plan, the purpose of which is to find out whether a simple or complex protein is in solution. If a complex protein is detected, how to establish (or exclude) that it is hemoglobin.
7. Explain the basis for dividing complex proteins into classes.
8. Give a brief description of all classes of complex proteins.
9. Remember the structural formulas of prosthetic groups of nucleic acids.
10. Characterize the nitrogenous bases that make up nucleic acids and list the differences between DNA and RNA (by localization, structure, functions).
11. Name the minimum information element in the structure of DNA and RNA.
12. Understand how the role of DNA and RNA is realized as sources of information.
13. Name two subgroups of chromoproteins and the differences between them.
14. To consolidate an understanding of the structure of hemoglobin (to study the components of the protein part and the components of the heme, as well as their role in the main function of hemoglobin).
LESSON 4 (final)
When preparing for the final lesson, check whether you have mastered the section "Structure and functions of proteins" using the following questions (use lecture materials and textbooks when preparing):
1. Formulate the concept of “Life”, including in the definition all the elements that are the subject of biochemistry.
2. Define the subject of biochemistry and list the issues that this science deals with.
3. Name the most important supramolecular formations of living things and the groups of molecules that make them up
4. Define the class “Proteins”
5. Define the class “Amino acids”.
6. Write the structural formulas of all tripeptides that can be built from histidine, alanine and valine.
7. Which of the following peptides are acidic, basic, or neutral and indicate the net electrical charge of each. pro-ser-ser; ala-pro-leu-thr; met-gly-ala; glu-his-ser; cys-lys-arg, glu-arg-lys; his-glu.
8. List the approaches to protein classification known to you
9. Name groups of proteins that differ in composition.
10. Name groups of proteins that differ in three-dimensional structure.
11. Name groups of complex proteins.
12. Continue the phrase “Loss of the native conformation under the influence of chemical, physical and other factors without violating the amino acid sequence is........”
13. List the types of chemical bonds that are broken during denaturation.
14. List in logical order the steps required to isolate proteins from tissues.
15. Draw the structural formulas of the nitrogenous bases that make up mononucleotides.
16. Draw the structural formulas of AMP, HMP, CMP, TMP and UMP.
17. Describe the method of connection between mononucleotides in a polynucleotide.
18. Name the differences between DNA and RNA in composition, structure, localization and function.
19. What type of protein is hemoglobin?
20. Name the structural features of globin.
21. Draw the structural formula of heme, name the connections between heme and globin.
22. What causes the diversity of functions of proteins?
23. List the biological functions of proteins.
Topic: “The nature and properties of enzymes” (lessons 5-9)
Target: study the chemical nature, functions and properties of biological catalysts - enzymes.
The meaning of the topic. Metabolism, an obligatory and most important feature of living organisms, is made up of many different chemical reactions, which involve compounds entering the body from the outside and compounds of endogenous origin. In the process of studying this section of the discipline, one learns that all chemical reactions in living things occur with the participation of catalysts, that catalysts in living things (enzymes or enzymes) are substances of a protein nature, that the properties of enzymes and their behavior depend on the characteristics of the environment.
When studying this section, information is also acquired about how the activity of enzymes is regulated in the whole organism, and general ideas are created about the connection of a number of pathological processes with changes in the activity or quantity of enzymes, information about the principles of quantitative characteristics of enzymes, and their use for diagnostic and therapeutic purposes.