The structure and chemical composition of bones. Bone
Bone tissue is distinguished by a number of very peculiar qualities that sharply distinguish it from all other tissues and systems of the human body and put it in a separate place. The main and main feature of bone tissue is its richness in mineral salts.
If we take the body weight of an adult as an average of 70 kg, then the bone skeleton weighs 7 kg, and together with the bone marrow - 10 kg (muscles - "meat" - weigh 30 kg). The bones themselves, by weight, are composed of 25% water, 30% organic matter, and 45% minerals. The water content and therefore the relative content of other ingredients also fluctuate. The amount of water is relatively very large in embryonic life, it decreases in childhood and gradually decreases as the child, adolescent and mature person grows and develops, reaching in old age the smallest ratio to the total weight. Bones dry out with age.
The organic composition of bones is formed mainly from proteins - proteins, mainly ossein, but some albumins, mucoid and other substances of a very complex chemical structure also enter into the complex organic part of the bone tissue.
What is the most interesting mineral composition of the bone substance? 85% of the salts are lime phosphate, 10.5% calcium carbonate, 1.5% magnesium phosphate, and the remaining 3% are sodium, potassium, impurities of chlorine and some rare elements for the human body. Calcium phosphate, therefore, constituting 19/20 of the content of the entire salt bone substance, forms 58% of the total weight of the bones.
Phosphate salts have a crystalline structure, and the crystals are located in the bone correctly, naturally. A very thorough study of the mineral skeleton of the bone substance, carried out in the 30s using the most advanced methods, primarily by X-ray structural analysis, showed that the inorganic human bone substance has the structure of phosphatite-apatite, namely hydroxyl-apatite. At the same time, it is interesting that the apatite in the bones (and in the teeth) of a person is close or even similar to the natural mineral apatite in dead nature. This identity of apatite of human bone and mining origin is also indicated by their comparative study in polarized light. Human bone apatite is also distinguished by the content of a small amount of chlorine or fluorine halide. Some structural analysts are of the opinion that in human bones, apatite is still associated with other chemical compounds, i.e. that the crystals of the inorganic bone substance are a mixture of two inorganic chemicals, one of which is close to apatite. It is believed that the most correct physico-chemical structure of bone apatite was deciphered by the Hungarian scientist St. Naray-Szabo. The most probable formula for the structure of the inorganic composition of the bone is: ZSA 3 (PO 4) 2 . CaX 2, where X is either Cl, F, OH, V2O, 1/2 SO 4, 1/2 CO 3, etc. There are also indications that apatite consists of two molecules - CaF. Ca 4 (PO 4) 3 or CaCl. Ca 4 (RO 4) 3.
Extremely interesting indications Reynolds (Reynolds) and others that under certain pathological processes, the bones lose their normal chemical apatite structure. This occurs, for example, in hyperparathyroid osteodystrophy (Recklinghausen's disease), while in Paget's disease, the apatite crystal structure is completely preserved.
Bone tissue is, albeit very ancient in phylogeny, but at the same time highly developed and exceptionally finely and in detail differentiated mesenchymal connective tissue, extremely complex in all its vital manifestations.
Changes in the bones in various pathological processes are infinitely varied; in each individual disease, in each individual bone, in each individual case, the pathoanatomical and pathophysiological, and consequently, the radiological picture has its own characteristics. All this vast variety of morbid phenomena is reduced, however, in the end only to some not so numerous elementary qualitative and quantitative processes.
Disease is, as is known, not only a perverted arithmetic sum of single normal phenomena; under pathological conditions, specific qualitative changes occur in the whole organism and in individual organs and tissues, for which there are no normal prototypes. Painfully altered bone also undergoes a deep qualitative metamorphosis. The periosteum, for example, forming a callus at the site of a diaphyseal fracture, begins to perform a new, normally uncharacteristic function, it produces cartilaginous tissue. A tumor of the bone is associated with the development of, for example, epithelial, myxomatous, giant cell and other formations that are as alien to normal bone histologically as chemically deposits of cholesterol in xanthomatosis or kerazine in Gaucher disease are unusual for it. The bone apparatus in rickets or Paget's restructuring acquires completely new physical, chemical, biological and other qualities, for which we are not able to find quantitative criteria for comparison in normal bone.
But these qualitative properties, specific for pathological processes in the bone substance, unfortunately, in themselves cannot be directly determined radiographically, they appear on radiographs only in the form of indirect, secondary symptoms. The power of radiology is not in their recognition and study. Only when the qualitatively altered tissue, in its quantitative determination, has reached the level of possible detection, does the X-ray method of research come into its own. With the help of impeccable experimental studies, Pauline Mack (Mack) proved that from the various components of bone tissue, the absorption of X-rays occurs by 95% due to the mineral composition (80% of the rays are retained by calcium and 15% by phosphorus), and only up to 5% the shadow image of the bones is due to the organic "soft" ingredient of the bone tissue. Therefore, due to the very nature of X-ray examination, in X-ray diagnostics of diseases of bones and joints, the assessment of quantitative changes in bone tissue comes to the fore. Scales cannot measure distance. The radiologist, with the help of his exceptionally valuable, but still one-sided method, is currently still forced to confine himself to an analysis of mainly two basic quantitative processes of the vital activity of the bone, namely, the creation of bone and its destruction.
IN compact bones: 20% organic matrix, 70% inorganic matter, 10% water. IN spongy bones: more than 50% - organic components, 33 - 40% - inorganic compounds, 10% - water.
Inorganic composition of bone tissue . In the human body ~ 1 kg of calcium, 99% of it is in the bones and teeth. Most of the Ca in the bones is constantly updated: during the day, the bones of the skeleton lose and again receive ~ 700 - 800 mg of Ca. Inorganic components of bone tissue are represented by:
hydroxyapatite crystals Ca 10 (RO 4) 6 (OH) 2, which are in the form of plates or sticks;
amorphous phosphate Ca - Ca 3 (RO 4) 2, which is considered a labile reserve of Ca and P ions.
At an early age, Ca 3 (PO 4) 2 predominates, and hydroxyapatite dominates in mature bone.
Na + , Mg 2+ , K + , Cl - etc.
Organic matrix of bone tissue: ~95% - type I collagen. It contains a lot of free ε-NH 2 -groups of Lys and oxylysin, as well as phosphates associated with Ser residues. The number of proteoglycans in mature dense bone is small. Among glycosaminoglycans, chondroitin-4-sulfate predominates and chondroitin-6-sulfate, keratan sulfate, and hyaluronic acid contain less; they participate in ossification. A lot of citrate (up to 90% of the total amount in the body): it is possible that citrate forms complex compounds with Ca and P salts and thereby increases their concentration in the tissue to a level at which crystallization and mineralization begin.
Throughout the life of the body, a constant restructuring of bone tissue continues. It is believed that the bone tissue of the human skeleton is almost completely rebuilt every 10 years. Bone metabolism, intake, deposition and excretion of Ca and P are regulated by parathyrin, calcitonin, calcitriol (1,25 (OH) 2 -D 3) (repeat!). Parathyrin activates osteoclasts, mineral (primarily Ca) and organic components enter the bloodstream. Calcitonin inhibits the activity of these cells, and the rate of bone formation increases. With a lack vitamin D involved in the synthesis of Ca-SB, slows down the formation of new bones and remodeling (renewal) of bone tissue. Chronic excess of vitamin D leads to bone demineralization. Vit.A: with a deficiency, bone growth stops due, probably, to a violation of the synthesis of chondroitin sulfate; with hypervitaminosis - bone resorption and fractures. Vit.C is needed for hydroxylation of Pro and Liz; with a deficiency: 1) abnormal collagen is formed, mineralization processes are disturbed; 2) the synthesis of glycosaminoglycans is disrupted: the content of hyaluronic acid in the bone tissue increases several times, and the synthesis of chondroitin sulfate slows down.
CHEMICAL COMPOSITION OF THE TOOTH.
The hard part of the tooth is represented by enamel, dentin and cementum. The cavity of the tooth is made of loose connective tissue - the pulp.
Enamel –
the hardest tissue in the human body, due to the high content of inorganic substances in it (up to 97%). Healthy enamel contains 1.2% organic matter and up to 3.8% water, which can be free and bound (in the form of a hydrated shell of apatite crystals).
Mineral base make up apatite crystals:
hydroxyapatite - 75%,
carbonate apatite - 19%,
chlorapatite - 4.4%,
fluorapatite - 0.66%,
non-apatite forms - less than 2%.
The general formula of apatites: A 10 (BO 4) X 2, where
A - Ca, Cr, Ba, Cd, Mg;
B—P, As, Si;
X - F, OH, Cl, CO 3 2-.
The crystals of different teeth are not the same; enamel crystals ~ 10 times larger than dentin and bone crystals. The composition of apatite may vary. “Ideal” apatite is Ca 10 (RO 4) 6 (OH) 2, i.e. ten-calcium, where the ratio Ca / P \u003d 1.67. This ratio can vary from 1.33 to 2.0, because substitution reactions are possible:
Ca 10 (RO 4) 6 (OH) 2 + Mg 2+ → Ca 9 Mg (RO 4) 6 (OH) 2 + Ca 2+
Such a substitution is unfavorable, because reduces enamel resistance. Another substitution, on the contrary, to the formation of a substance with greater resistance to dissolution:
Ca 10 (RO 4) 6 (OH) 2 + F - → Ca 10 (RO 4) 6 F (OH) + OH -
hydroxyfluorapatite
However, when exposed to high concentrations of F on hydroxyapatite, the reaction proceeds differently:
Ca 10 (RO 4) 6 (OH) 2 + 20 F - → 10 CaF 2 + 6 RO 4 3- + 2 OH -
The resulting Ca fluoride quickly disappears from the surface of the teeth.
There may be vacancies in the crystal lattice of hydroxyapatites, which increases the ability of crystals to surface reactions. For example, if decacalcium hydroxyapatite has a total neutral charge, then octassium hydroxyapatite is negatively charged: (Ca 8 (PO 4) 6 (OH) 2) 4- and is able to bind counterions.
Each hydroxyapatite crystal is covered with a hydrate shell (~1 nm). The penetration of various substances into a hydroxyapatite crystal occurs in 3 stages:
Stage 1 - ion exchange between the solution surrounding the crystal and the hydration shell, in which phosphate, carbonate, citrate, Ca, Sr can accumulate as a result. Some ions (K + , Cl -) can easily enter the hydrated layer and leave it, other ions (Na + , F -), on the contrary, pass into the hydroxyapatite crystal. The 1st stage is a very fast process, lasting several minutes, based on the diffusion process;
Stage 2 - exchange of ions between the hydration shell and the surface of the hydroxyapatite crystal. Runs slowly (several hours). The surface ions of the crystal break off, go into the hydrate shell, and others from the hydrate layer take their place. Phosphate, Ca, F, carbonate, Sr, Na penetrate into the surface of the hydroxyapatite crystal;
Stage 3 - the introduction of ions from the surface deep into the crystal, i.e. intracrystalline exchange. Ca, Sr, phosphate, F can penetrate inside the crystal. It flows for a long time, days - months.
Thus, hydroxyapatite crystals are unstable, their composition and properties change depending on the solution surrounding the crystal. It is used in practical dentistry.
Most of the hydroxyapatite crystals in enamel are oriented and ordered in a certain way in the form of more complex formations - enamel prisms, each of which consists of thousands and millions of crystals. Enamel prisms are collected in bunches.
organic matter enamels are represented by proteins, peptides, free amino acids (Gli, Val, Pro, Opr), fats, citrate, carbohydrates (galactose, glucose, mannose, glucuronic acid, fucose, xylose).
Enamel proteins are divided into 3 groups:
I - water-soluble proteins; molecular weight - 20000, not associated with mineral substances;
II - calcium-binding protein (Ca-SB): molecular weight 20,000; 1 mol of Ca-SB can bind 8-10 Ca ions and form an insoluble complex with Ca 2+ in a neutral medium, like di-, tri- and tetramers weighing 40-80 thousand. Phospholipids are involved in the formation of Ca-SB aggregates with Ca. In an acid medium, the complex decomposes;
III - proteins that are insoluble in EDTA and HCl (even in 1N solution). Insoluble enamel proteins are similar in amino acid composition to collagen, but not identical to it: the enamel protein contains less than collagen, Pro and Gly, there is almost no ODA, but there are many carbohydrates associated with it.
The role of the protein: 1) surrounding apatites, the protein prevents the contact of acid with them or softens its effect, i.e. delay the demineralization of this layer;
2) are a matrix for mineralization and remineralization (in the mechanism of biological calcification).
Suggested functional-molecular model of the enamel structure, according to which Ca-SB molecules, interconnected by calcium bridges, form a three-dimensional network; In this case, Ca can be free or enter the structure of hydroxyapatite. This mesh is attached through Ca to the skeleton (skeleton, soft skeleton of enamel), which is formed by an insoluble protein. Ca-SB functional groups capable of binding Ca, and this is a phosphate in the composition of either phosphoserine or protein-bound phospholipids; The COOH groups of Glu, Asp, and aminocitrate serve as centers (points) of nucleation during crystallization. Thus, proteins provide orientation during crystallization, strict order, uniformity and sequence of enamel formation. The degree of mineralization depends on salivation, blood supply, supersaturation of Ca 2+ and phosphate, on the pH of the medium, etc.
Dentine
makes up the bulk of the tooth. (The crown part of the tooth is covered with enamel, the root part is covered with cement). Composition: up to 72% - inorganic substances (mainly phosphate, carbonate, calcium fluoride), ~ 28% - organic substances (collagen) and water. Dentin is built from the ground substance and tubules passing through it, in which there are processes of odontoblasts and the endings of nerve fibers penetrating from the pulp. The main substance contains bundled collagen fibers and an adhesive substance, which contains a large amount of mineral salts. The process of dentin formation occurs during the entire period of tooth functioning in the presence of a viable pulp. The dentin formed after a tooth has erupted is called secondary dentin. It is characterized by a lower degree of mineralization and a high content of collagen fibrils. The dentinal tubules can circulate dentinal fluid and supply nutrients. The intertubular substance is represented by hydroxyapatite crystals, has a high density and hardness. There are many fibrils in the cytoplasm of odontoblasts, there are free ribosomes, lipid granules.
The intercellular organic matrix of a compact bone is about 20%, inorganic substances - 70% and water - 10%. Cancellous bone is dominated by organic components, which account for more than 50%, while inorganic compounds account for 33-40%. The amount of water is approximately the same as in compact bone.
Organic bone matrix. Approximately 95% of the organic matrix is collagen type I. This type of collagen is also found in tendons and skin, but bone tissue collagen has some characteristics. It contains somewhat more hydroxyproline, as well as free amino groups of lysine and oxylysine residues. This causes the presence of more cross-links in collagen fibers and their greater strength. Compared to the collagen of other tissues, bone collagen is characterized by an increased content of phosphate, which is mainly associated with serine residues.
Proteins of a non-collagen nature are represented by glycoproteins, the protein components of proteoglycans. They take part in the growth and development of the bone, the process of mineralization, water-salt metabolism. Albumins are involved in the transport of hormones and other substances from the blood.
The predominant non-collagenous protein is osteocalcin. It is present only in bones and teeth. It is a small (49 amino acid residues) protein, also called bone glutamine protein or gla protein. Three residues were found in the osteocalcin molecule
γ-carboxyglutamic acid. Due to these residues, it is able to bind calcium. Vitamin K is necessary for the synthesis of osteocalcin (Fig. 34).
Rice. 34. Post-translational modification of osteocalcin
The composition of the organic matrix of bone tissue includes glycosaminoglycans, the main representative of which is chondroitin-4-sulfate. Chondroitin-6-sulfate, keratan sulfate and hyaluronic acid are contained in small amounts. Ossification is accompanied by a change in glycosaminoglycans: sulfated compounds give way to non-sulfated ones. Glycosaminoglycans are involved in the binding of collagen to calcium, the regulation of water and salt metabolism.
Citrate is essential for bone mineralization. It forms complex compounds with calcium and phosphorus salts, making it possible to increase their concentration in the tissue to a level at which crystallization and mineralization can begin. Also takes part in the regulation of calcium levels in the blood. In addition to citrate, succinate, fumarate, malate, lactate and other organic acids were found in bone tissue.
The bone matrix contains a small amount of lipids. Lipids play an essential role in the formation of crystallization nuclei during bone mineralization.
Osteoblasts are rich in RNA. The high content of RNA in bone cells reflects their activity and constant biosynthetic function.
Inorganic composition of bone tissue.
At an early age, amorphous calcium phosphate Ca 3 (PO 4) 2 predominates in bone tissue. In a mature bone, crystalline hydroxyapatite Ca 10 (PO 4) 6 (OH) 2 becomes predominant (Fig. 35). Its crystals are in the form of plates or sticks. Usually, amorphous calcium phosphate is considered as a labile reserve of Ca 2+ and phosphate ions.
The composition of the bone mineral phase includes ions of sodium, magnesium, potassium, chlorine, etc. In the crystal lattice of hydroxyapatite, Ca 2+ ions can be replaced by other divalent cations, while anions other than phosphate and hydroxyl are either adsorbed on the surface of crystals or dissolved in hydrate shell of the crystal lattice.

Rice. 35. The structure of a hydroxyapatite crystal
bone metabolism characterized by two opposite processes: the formation of new bone tissue by osteoblasts and the resorption (degradation) of old osteoclasts. Normally, the amount of newly formed tissue is equivalent to the destroyed one. The bone tissue of the human skeleton is almost completely rebuilt within 10 years.
Bone formation
On 1 stage osteoblasts first synthesize proteoglycans and glycosaminoglycans that form the matrix, and then produce bone collagen fibrils, which are distributed in the matrix. Bone collagen is the matrix for the mineralization process. A necessary condition for the process of mineralization is the supersaturation of the medium with calcium and phosphorus ions. The formation of crystals of the mineral backbone of the bone is triggered
Ca-binding proteins on a collagen matrix. Osteocalcin is strongly associated with hydroxyapatite and is involved in the regulation of crystal growth by binding Ca 2+ in bones. Electron microscopy studies have shown that the formation of a mineral crystal lattice begins in zones located in regular intervals between collagen fibrils. The formed crystals in the collagen zone then, in turn, become mineralization nuclei, where hydroxyapatite is deposited in the space between the collagen fibers.
On 2 stage in the mineralization zone, with the participation of lysosomal proteinases, degradation of proteoglycans occurs; oxidative processes intensify, glycogen breaks down, the required amount of ATP is synthesized. In addition, the amount of citrate required for the synthesis of amorphous calcium phosphate increases in osteoblasts.
As bone tissue mineralizes, hydroxyapatite crystals displace not only proteoglycans, but also water. Dense, fully mineralized bone is practically dehydrated.
The enzyme alkaline phosphatase is involved in mineralization. One of the mechanisms of its action is a local increase in the concentration of phosphorus ions to the saturation point, followed by the processes of fixation of calcium-phosphorus salts on the organic matrix of the bone. When bone tissue is restored after fractures, the content of alkaline phosphatase in the callus increases sharply. In violation of bone formation, a decrease in the content and activity of alkaline phosphatase in bones, plasma and other tissues is observed.
Calcification inhibitor is inorganic pyrophosphate. A number of researchers believe that the process of collagen mineralization in the skin, tendons, and vascular walls is hindered by the constant presence of proteoglycans in these tissues.
The processes of modeling and remodeling ensure the constant renewal of bones, as well as the modification of their shape and structure. Modeling (new bone formation) takes place mainly in childhood. Remodeling is the dominant process in the adult skeleton; in this case, only a separate section of the old bone is replaced. Thus, under physiological and pathological conditions, not only the formation, but also the resorption of bone tissue occurs.
bone tissue catabolism
Almost simultaneously, "resorption" of both mineral and organic structures of bone tissue takes place. During osteolysis, the production of organic acids increases, which leads to a shift in pH to the acid side. This contributes to the dissolution of mineral salts and their removal.
The resorption of the organic matrix occurs under the action of lysosomal acid hydrolases, the spectrum of which in the bone tissue is quite wide. They are involved in the intracellular digestion of fragments of resorbable structures.
In all diseases of the skeleton, there are violations of the processes of bone remodeling, which is accompanied by the occurrence of deviations in the level of biochemical markers.
There are common markers of new bone formation such as bone specific alkaline phosphatase, plasma osteocalcin, procollagen I, plasma peptides. to biochemical bone resorption markers include urinary calcium and hydroxyproline, urinary pyridinoline, and deoxypyridinoline, which are derivatives of transverse collagen fibers specific to cartilage and bone.
Factors that affect bone metabolism are hormones, enzymes and vitamins.
The mineral components of bone tissue are practically in a state of chemical equilibrium with calcium and phosphate ions in blood serum. Parathyroid hormone and calcitonin play an important role in the regulation of the intake, deposition and release of calcium and phosphate.
The action of parathyroid hormone leads to an increase in the number of osteoclasts and their metabolic activity. Osteoclasts contribute to the accelerated dissolution of mineral compounds contained in the bones. Thus, there is an activation of cellular systems involved in bone resorption.
Parathyroid hormone also increases the reabsorption of Ca 2+ ions in the renal tubules. The overall effect is manifested in an increase in the level of calcium in the blood serum.
The action of calcitonin is to reduce the concentration of Ca 2+ ions due to its deposition in bone tissue. It activates the enzyme system of osteoblasts, increases bone mineralization and reduces the number of osteoclasts in the area of action, i.e., it inhibits the process of bone resorption. All this increases the rate of bone formation.
Vitamin D is involved in the biosynthesis of Ca 2+ -binding proteins, stimulates the absorption of calcium in the intestine, increases the reabsorption of calcium, phosphorus, sodium, citrate, amino acids in the kidneys. With a lack of vitamin D, these processes are disrupted. Taking excessive amounts of vitamin D for a long time leads to demineralization of bones and an increase in the concentration of calcium in the blood.
Corticosteroids increase the synthesis and secretion of parathyroid hormone, enhance bone demineralization; sex hormones accelerate maturation and shorten the period of bone growth; thyroxine enhances tissue growth and differentiation.
The effect of vitamin C on bone metabolism is primarily due to the effect on the process of collagen biosynthesis. Ascorbic acid is a cofactor for prolyl and lysyl hydroxylases and is necessary for the hydroxylation of proline and lysine. Vitamin C deficiency also leads to changes in the synthesis of glycosaminoglycans: the content of hyaluronic acid in bone tissue increases several times, while the biosynthesis of chondroitin sulfates slows down.
With a lack of vitamin A, there is a change in the shape of the bones, a violation of mineralization, growth retardation. It is believed that this fact is due to a violation of the synthesis of chondroitin sulfate. High doses of vitamin A lead to excessive bone resorption.
With a lack of B vitamins, bone growth slows down, which is associated with a violation of protein and energy metabolism.
Features of dental tissue
The main part of the tooth is dentine. The protruding part of the tooth, the crown, is covered enamel, and the root of the tooth is covered dental cement. Cement, dentin and enamel are built like bone tissue. The protein matrix of these tissues consists mainly of collagens and proteoglycans. The content of organic components in cement is about 13%, in dentine - 20%, in enamel - only 1-2%. The high content of minerals (enamel - 95%, dentin - 70%, cement - 50%) determines the high hardness of the dental tissue. The most important mineral component is hydroxyapatite [Ca 3 PO 4) 2 ] 3 Ca(OH) 2 . It also contains carbonate apatite, chlorapatite and strontium apatite.
The enamel covering the tooth is semi-permeable. It participates in the exchange of ions and molecules with saliva. Enamel permeability is affected by the pH of saliva, as well as a number of chemical factors.
In an acidic environment, the tooth tissue is attacked and loses its hardness. Such a common disease caries, is caused by microorganisms living on the surface of the teeth and releasing organic acids as a product of anaerobic glycolysis, leaching Ca 2+ ions from the enamel.
Control questions
1. Name the main organic components of bone tissue.
2. What inorganic compounds are included in the bone tissue?
3. What is the difference between biochemical processes occurring in osteoclasts and osteoblasts?
4. Describe the process of bone formation.
5. What factors influence the formation of bone tissue and its metabolism?
6. What substances can be biochemical markers of processes occurring in bone tissue?
7. What are the features of the biochemical composition of dental tissue?
Literature
1. Berezov, T.T. Biological chemistry. / T.T. Berezov, B.F. Korovkin. - M.: JSC "Publishing house" Medicine "", 2007. - 704 p.
2. Biochemistry. / Ed. E.S. Severin. - M.: GEOTAR-Media, 2014. -
768 p.
3. Biological chemistry with exercises and tasks. / Ed. E.S. Severin. - M.: GEOTAR-Media, 2013. - 624 p.
4. Zubairov, D.M. Guide to laboratory studies in biological chemistry. / D.M. Zubairov, V.N. Timerbaev, V.S. Davydov. - M.: GEOTAR-Media, 2005. - 392 p.
5. Shvedova, V.N. Biochemistry. /V.N. Shvedova. – M.: Yurayt, 2014. – 640 p.
6. Nikolaev, A.Ya. Biological chemistry. / AND I. Nikolaev. - M.: Medical Information Agency, 2004. - 566 p.
7. Kushmanova O.B. Guide to laboratory studies in biological chemistry. / ABOUT. Kushmanova, G.I. Ivchenko. - M. - 1983.
8. Lehninger, A. Fundamentals of biochemistry / A. Lehninger. - M., Mir. - 1985.
9. Murray, R. Human biochemistry. / R. Murray, D. Grenner, P. Meyes, V. Rodwell. - T. 1. - M.: Mir, 1993. - 384 p.
10. Murray, R. Human biochemistry. / R. Murray, D. Grenner, P. Meyes, V. Rodwell. - T. 2. - M.: Mir, 1993. - 415 p.
Vestn. Ohm. university 2015. No. 4. S. 39-44.
UDC 54.062, 543.544.5.068.7
S.A. Gerk, O.A. Golovanova
A comparative study of the micro- and macro-elemental composition of human bone tissues in the "normal" with the content of elements in bone samples damaged due to coxarthrosis, as well as in physiogenic (dentin and tooth enamel) and pathogenic (salivary, dental and kidney stones) biominerals was carried out. It is shown that in the "normal" bone tissue in terms of mineral composition is closest to dentin and tartar. It has been established that in human bone tissues with coxarthrosis, the value of the atomic ratio Ca / P and the content of elements: copper, tin, iron, manganese, strontium and chromium (in some cases) change. The relationship between the concentration series of microelements Zn > Sr > Fe of the affected bone tissue with the series for dental and kidney stones was revealed.
Key words: elemental composition, physiogenic and pathogenic mineralization, bones, coxarthrosis, spectroscopy. *2
Introduction
Bone tissue belongs to highly specialized physiogenic biominerals and is a biochemical system with a multicomponent composition and complex structure. Thanks to such a structural organization, this organo-mineral aggregate (hereinafter referred to as OMA) ensures the normal course of metabolism (metabolism) in the human body as a whole. At the same time, being in constant contact with biological fluids, bone tissue is a place of deposition of macro- and microelements. It is known that the elements are not synthesized in the body, but come with food, water, air and play an important role in bone remodeling. So, summarizing the literature data on the role and degree of participation of trace elements in bone formation, they can be divided into five groups: 1) bone mineralization activators - Cu, Mn, F, Si, V;
2) inhibitors of bone mineralization - Sr, Cd, Be, Fe; 3) bone resorption activators - Mg, Zn, Ba; 4) elements involved in the synthesis of organic substances - Zn, Be, Cu, Mn, Si; 5) activators of bone cells and enzymes - Mg, Zn, Be and their inhibitors - Mo. A change in the content of elements in bone tissue (excess or deficiency), primarily calcium and phosphorus, leads to disruption of metabolic processes and is the cause of various osteoarticular diseases, dental pathologies and pathogenic mineral formation - the formation of salivary, dental, kidney and other stones. However, despite a significant number of works that describe the role of macro- and microelements in physiological processes, there are still debatable data on the elemental composition of bone tissues, including those under conditions of pathological development.
The urgency of this problem is also increasing in connection with the continuing difficult environmental situation of natural objects (sources of elements entering the human body) of industrial metropolitan cities, namely: excessive emissions of industrial waste into the atmosphere, increased exploitation of soils, irrational use of natural resources and pollution of water sources. So, today the water of many rivers in Russia has become practically unsuitable for drinking due to the content of organic substances of synthetic origin (surfactants, PAHs, dioxins), oil, oil products and salts of heavy metals exceeding the MPC.
Purpose of the work: to study the features of the elemental composition of human bone tissue in the "normal" in comparison with pathogenic OMA and in bone diseases (for example, coxarthrosis).
* The work was supported in part by the Presidential Grants Council Russian Federation, project No. SP-933.2015.4, Russian Foundation for Basic Research (grant no. 15-29-04839 ofi_m).
© S.A. Gerk, O.A. Golovanova, 2015
S.A. Gerk, O.A. Golovanova
Objects and methods of research
The work is a continuation of the study of the collection of femoral heads of men and women of the Omsk region aged 30 to 79 years, removed due to coxarthrosis. Unaffected samples were used as control samples of bone tissue, which were taken in accordance with the Order of the Ministry of Health of the USSR dated July 21, 1978 No. 694 “On approval of the instructions for the production of a forensic medical examination, the regulation on the bureau of forensic medical examination and other regulatory acts on forensic medical examination” (p. 2.24), federal laws of January 12, 1996 No. 8-FZ “On burial and funeral business” (p. 3) and of May 31, 2001 No. 73-FZ “On state judicial -expert activity in the Russian Federation” (p. 14, 16). To study the dynamics of the disease, three horizontal sections were obtained from the femoral heads: upper, middle, and lower (the alternation order is given in the direction of hyaline cartilage - femur), which were further analyzed in the form of dry powder samples. The average composition of different affected plates was compared with each other and with control samples.
by the power of the following spectral analysis methods: calcium ions - the method of atomic absorption spectroscopy (AAS) on the AAS 1N spectrometer according to GOST 26570-95; total phosphorus - spectrophotometric method on the automated line "Contiflo" (GOST 26657-97); the remaining elements were measured by inductively coupled plasma mass spectroscopy (ICP-MS) on an ELAN 9000 spectrophotometer. The element ion concentrations were calculated from calibration curves using standard solutions. The limits of detection of elements by spectrophotometry and AAS were 10-6 wt. %, for ICP-MS - 10-9 -10--13 wt. %.
Statistical processing of the obtained data was carried out by Student's method for a confidence probability P = 0.95, based on the assumption of their distribution according to the normal law (Statistic Soft 2006 software package).
Results and its discussion
An analysis of literary sources showed that the data on the quantitative content of elements in bone tissue are quite contradictory, which is due to the specific composition of different bones, their type (Table 1), age characteristics of a person (Table 2), environmental conditions (climate, technogenic impact) , the nature of nutrition, etc.
Table 1
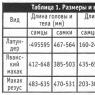
Studied bone Mn Al Cu Ti V
Peroneal 0.173 ± 0.030 0.113 ± 0.017 0.086 ± 0.030 0.062 ± 0.006 0.006 ± 0.004
Tibial 0.184 ± 0.024 0.106 ± 0.024 0.084 ± 0.022 0.063 ± 0.006 0.006 ± 0.0007
Femoral 0.220 ± 0.048 0.117 ± 0.034 0.040 ± 0.012 0.078 ± 0.010 0.006 ± 0.001
Average 0.192 ± 0.031 0.112 ± 0.016 0.070 ± 0.020 0.068 ± 0.008 0.006 ± 0.001
table 2
Trace elements Bone age
embryos from 16-17 to 21 weeks from one day to 19 years from 20 to 40 years from 50 to 83 years
Fe 215.8 146.2 132.8 119.3
Si 23.8 25.3 22.4 16.4
Al 5.96 6.45 7.42 8.09
Pb 4.48 3.03 7.09 1.04
Cu 2.86 1.64 1.42 1.24
Sr 1.27 2.73 1.48 6.78
Ti 1.01 1.13 1.02 1.25
Mn 0.99 1.08 1.17 1.24
A comparative study of the literature and experimental data made it possible to establish that the main macronutrients of the bone, the content of which is more than 10-3% of the body weight, are calcium, phosphorus, sodium, potassium, and magnesium; elements with a mass composition from 10-3 to 10-6% include zinc, manganese, copper, nickel, and others (Tables 3 and 4). It can be seen that physiogenic OMA (bones, teeth) differ significantly in their macroelement composition from pathogenic phosphate-type calculi, which are most common in
human body (dental, salivary and kidney stones). The interval of variation of elements in bone and dental tissue is narrower, obviously due to the regular nature of the formation of physiogenic biominerals and the lesser influence of endogenous factors on this process. It can be conditionally noted that the bone tissue in terms of mineral composition (Ca, P, Na, K, Mg) is closest to the physiogenic OMA - dentin and to pathogenic biominerals - tartar, which may indicate the similarity of the compositions of mineral-forming media and / or mechanisms of their formation. .
Elemental composition of human bone tissue in normal and pathological conditions
Table 3
Macroelement composition of physiogenic (bone tissue, enamel and dentin of the tooth) and pathogenic (tooth, salivary, kidney stones) OMA of the phosphate type, wt. %
Component Bone tissue Enamel Dentin Tartar 9; 25] Salivary Stones Kidney Stones d
"normal" for coxarthrosis
Ca/P 1.37 1.77 - 0.89 ± 0.04 1.81 ± 0.01 1.63 1.6-1.69 1.61 1.64-1.65 1.49-2, 04 1.49-1.79 - 1.67
Na 0.70 0.90 0.50 0.44 ± 0.02 0.46 ± 0.14 0.50-0.90 0.25-0.90 0.60 0.7 0.37-0, 88 0.28-0.95 0.1-2.43 -
Mg 0.55 0.72 0.30 0.19 ± 0.007 0.22 ± 0.01 0.07-0.44 0.25-0.56 1.23 0.8-1.0 0.32- 0.50 0.20-0.24 1.5-84.58 -
K 0.03 0.03 0.20 0.058 ± 0.013 0.028 ± 0.013 0.001-0.008 0.05-0.30 0.05 0.02-0.04 0.11-0.13 0.03-0.12 0.07-4.05 -
Note: "-" - no data.
Table 4
Elemental composition of physiogenic (bone tissue, enamel, dentin of the tooth) and pathogenic (tooth, salivary, kidney stones) OMA of the phosphate type, -10-4 wt. %
Element Bone tissue Enamel Dentin Dental stones Salivary stones Kidney stones
1; . Perhaps the dominant substitution of ions in the structure of bone apatite in this case is the anionic substitution of phosphate tetrahedra, which is one of the reasons for the decrease in the crystallization of hydroxyapatite in bone tissues.
As in the case of macroelement composition, the content of microelements in bone tissue differs significantly from pathogenic OMA (Table 4). The composition of pathogenic biominerals includes the largest number of microelements, which is once again confirmed
waiting for a spontaneous and physiologically uncontrolled mechanism of their formation. All elements in pathogenic calculi are contained in a smaller amount than in the bones. Unlike other physiogenic minerals, bone tissue is second only to enamel in terms of the content of Pb, Si, Zn, Sr, Ag. At the same time, it contains more copper (13 times) and barium (5 times). Compared to dentin, this biomineral is the richest in almost all trace elements, with the exception of zinc and silver.
Ranking series of trace elements, the content of which is 0.0050.2 wt. %, according to the increase in their concentrations are as follows (Table 4): for bone tissue - Fe > > Cu > Ba > Pb > Si > Zn > Sr > Ni > Al > Mn; tartar - Zn > Sr > Fe > Ti > Cr; for salivary stones - Ti > V > Cr > Fe > I; for kidney stones - Sr > Zn > Fe. It can be seen that in comparison with bone tissue in pathogenic biominerals, the number of elements in the row, the content of which in OMA is not less than 0.005 wt. %, decreases by 2 times (for salivary and dental stones) and 3 times (for kidney stones). The remaining elements in pathogenic aggregates are presented in smaller quantities than in the bone. Iron is present in all rows, Sr and Zn are also present in large quantities in the renal and dental formations, and new elements Cr and Ti appear in the salivary and renal formations. The data presented indicate the different degree of participation of elements in pathogenic and physiogenic mineralization. The primary role in the mineralization of various nature belongs to iron, strontium and zinc. Microelements such as Cr and Ti are involved in pathogenic OMA.
The femoral heads of the collection we studied, in contrast to the literature data, contain trace elements in small amounts (Table 4). So, the concentration series of elements, the content of which exceeds 0.005 wt. %, consist of two and three elements: in the "normal" - Zn > Sr and with coxarthrosis - Zn > Sr > Fe. Such a sequence of elements in case of bone tissue damage correlates with rows for dental and kidney stones, which may indicate a pathological course of the process of bone tissue mineralization in coxarthrosis.
It was revealed that in the affected upper sections of bone tissues of persons of the first and second age groups (30-49 and 50-59 years old), compared with control samples, the content of copper ions was increased by 3 times, tin by 4 times, iron by 11 times, manganese by 17 times and chromium (in a number of samples) 18 times (Fig. 2). Also, in contrast to the "norm" in damaged samples, a slight decrease in the amount of strontium ions can be noted.
Elemental composition of human bone tissue in normal and pathological conditions
Therefore, the results obtained indicate a violation of the processes of bone remodeling in coxarthrosis. On the one hand, the content of elements that have an activating effect on bone mineralization (Cu and Mn) increases, on the other hand, the amount of microelements that accelerate the rate of bone resorption (Fe and Sn) changes. Elevated concentrations of the toxic element chromium in a number of samples also indicate the destructive (degenerative) nature of metabolism in this disease. The role of tin in bone metabolism has not yet been studied.
In the samples of bone tissues of persons of the third and fourth categories (60-69 and 70-79 years old), it was not possible to establish certain patterns in the change in the content of trace elements in pathology, which may be associated with the aging of bone tissue and the presence of concomitant diseases in this age range.
Thus, it was found in the work that in diseases caused by impaired Ca/P metabolism, such as coxarthrosis, the content of the following elements in human bone tissues changes: copper, tin, iron, manganese, strontium and chromium (in some cases). With this damage, an increase in the value of the Ca/P coefficient was revealed, mainly due to a decrease in the content of total phosphorus.
The composition of bone tissue, in contrast to pathogenic OMA, includes a smaller number of microelements, the content of which depends on the degree of bone mineralization.
pattern. The relationship between the concentration series of microelements Zn > Sr > Fe of the affected bone tissue with the series for dental and kidney stones was revealed, which may indicate a pathological course of bone mineralization.
It is shown that under the conditions of the physiological "norm" bone tissue in terms of mineral composition is closest to the physiogenic OMA - dentin and to pathogenic biominerals - tartar.
The obtained data can be used in studying the processes of bone mineralization under model conditions in order to develop effective therapeutic and prophylactic methods for restoring bone tissues in osteoarticular diseases.
LITERATURE
Avitsyn A.P., Zhavoronkov A.A., Rish M.A., Strochkova L.S. Human trace elements. M., 1991. 496 p.
Zatsepin S.T. Bone pathology in adults. M., 2001. 640 p.
Luneva S. N. Biochemical changes in the tissues of the joints in degenerative-dystrophic diseases and methods of biological correction: dis. ... Dr. Biol. Sciences. Tyumen, 2003. 297 p.
Erokhin A. N., Isakov B. D., Nakoskin A. N. Features of microelement composition of bone tissue in transosseous distraction osteosynthesis by the Ilizarov method in high altitude conditions (experimental study) // Saratov Journal of Medical Scientific Research.
2014. No. 10 (1). pp. 119-123.
Novikov M. I. Dynamics of accumulation of biogenic macro- and microelements in the bone tissue of dogs in postnatal ontogenesis and under conditions of transosseous distraction osteosynthesis: dis. ... cand. biol. Sciences. N. Novgorod, 2008. 137 p.
Lemesheva S.A. Chemical composition, properties of bone apatite and its analogues: dis. ... cand. chem. Sciences. M., 2010. 177 p.
Prokhonchukov A. A., Zhizhina N. A., Tigranyan R. A. Bone tissue homeostasis in normal and under extreme conditions. M., 1984. 200 p.
Golovanova OA, Borbat VF Kidney stones. M., 2005. 171 p.
Golovanova OA Biomineralogy of urinary, bile, dental and salivary stones from the human body: dis. ... Dr. geol.-mineral. Sciences. Tomsk, 2009. 240 p.
Aleksandrova T. V., Nakhaeva V. I. Genotoxic analysis of water samples of a natural source of drinking water from the Om River for gene and chromosomal mutations // Modern problems of science and education. 2014. No. 6. URL: http://www.science-education.ru/120-15369.
GOST 26570-95. Feed, compound feed, compound feed raw materials. Methods for determining calcium. M., 2000.
GOST 26657-97. Feed, compound feed, compound feed raw materials. Method for determination of phosphorus content. M., 2000.
S.A. Gerk, O.A. Golovanova
Nakoskin A.N. Age-related changes and gender differences in the biochemical composition of human bone tissue: dis. ... cand. biol. Sciences. Kurgan, 2004. 111 p.
Lundager Madsen H. E., Abbona F., Barrese E. Effects of cadmium on crystallization of calcium phosphates // Crystal Research and Crystal Technology. 2004 Vol. 39. No. 3. P. 235-239.
Voinar A. O. Importance of trace elements in the human and animal organisms. M., 1955. 24 p.
Enoka R. M. Fundamentals of kinesiology: Per. from English. Kyiv: Olympic Literature, 1998. 399 p.
Gilinskaya L. G., Zanin Yu. N., Nazmov V. P. Typomorphism of CO2-, CO3- and CO33- paramagnetic radicals in natural carbonate-apatites // Geology and Geophysics. 2002. T. 43. No. 3. S. 297303.
Matveeva E. L. Biochemical changes in the synovial fluid during the development of degenerative-dystrophic processes in the knee joint: author. dis. ... Dr. Biol. Sciences. Tyumen, 2007. 24 p.
Verbova A.F. Status of bone tissue and calcium-phosphorus metabolism in phosphorus production workers // Kazan Medical Journal. 2002. V. 83. No. 2. S. 148-150.
Newman U, Newman M. Mineral bone metabolism / per. from English. O. Ya. Tereshchenko, L. T. Tutochkina; ed. N. N. Demina. M., 1961.270 p.
Legeros R. Z. Calcium phosphates in oral biology and medicine. Karger, 1991. 221 p.
Korzh A. A., Belous A. M., Panov E. Ya. Reparative bone regeneration. M., 1972. 215 p.
Pilat T. L. Dental stone and its influence on periodontal tissues // Dentistry. 1984. No. 3. S. 88-90.
Tkalenko A.F. Influence of physicochemical characteristics of saliva, salivary and dental deposits on the outcome of treatment of patients with salivary stone disease: author. dis. . cand. honey. Sciences. M., 2004. 26 p.
Kiseleva DV Peculiarities of composition, structure and properties of a number of phosphate and carbonate biomineral formations: dis. ... geol.-mineral. Sciences. Ekaterinburg, 2007. 197 p.
LeGeros R. Z. Formation and transformation of calcium phosphates: relevance to vascular calcification // Zeitschrift fur Kardiologie. 2001. Supplement Band 90, pp. 116-124.
Smolegovsky A. M. History of crystal chemistry of phosphates. M., 1986. 263 p.
Barinov S. M., Komlev V. S. Bioceramics based on calcium phosphates. M., 2005. 204 p.
Christoffersen M. R., Seierby N., Zunic T. B., Chris-toffersen J. Kinetics of dissolution of triclinic calcium pyrophosphate dehydrate crystals // Journal of Crystal Growth. 1999 Vol. 203. R. 234-243.
The composition of the fresh bone of an adult includes water - 50%, fat - 16%, other organic substances - 12%, inorganic substances - 22%.
Defatted and dried bones contain approximately 2/3 inorganic and 1/3 organic matter. In addition, bones contain vitamins A, D and C.
Organic bone tissue ossein- gives them elasticity. It dissolves when boiled in water, forming bone glue. The inorganic content of bones is represented mainly by calcium salts, which, with a small admixture of other mineral substances, form hydroxyapatite crystals.
The combination of organic and inorganic substances determine the strength and lightness of bone tissue. Thus, at a low specific gravity of 1.87, i.e. twice the specific gravity of water, the strength of bone exceeds the strength of granite. The femur, for example, when compressed along the longitudinal axis, can withstand loads of more than 1500 kg. If the bone is fired, then the organic matter burns out, while the inorganic matter remains and retains the shape of the bone and its hardness, but such a bone becomes very brittle and crumbles when pressed. On the contrary, after soaking in a solution of acids, as a result of which mineral salts dissolve, and organic matter remains, the bone also retains its shape, but becomes so elastic that it can be tied into a knot. Consequently, the elasticity of the bone depends on ossein, and its hardness depends on mineral substances.
The chemical composition of bones is associated with age, functional load, and the general condition of the body. The greater the load on the bone, the more inorganic substances. For example, the femur and lumbar vertebrae contain the largest amount of calcium carbonate. With increasing age, the amount of organic in-in decreases, and inorganic increases. In small children, there is relatively more ossein, respectively, the bones are very flexible and therefore rarely break. On the contrary, in old age the ratio of organic and inorganic substances changes in favor of the latter. Bones become less elastic and more fragile, as a result of which bone fractures are most often observed in the elderly.
Bone classification
According to the shape, function and development of the bones are divided into three parts: tubular, spongy, mixed.
tubular bones are part of the skeleton of the limbs, playing the role of levers in those parts of the body where movements on a large scale predominate. Tubular bones are divided into long- humerus, forearm bones, femur, lower leg bones and short- bones of the metacarpus, metatarsus and phalanges of the fingers. Tubular bones are characterized by the presence of a middle part - diaphysis, containing a cavity (bone marrow cavity), and two expanded ends - epiphyses. One of the epiphyses is located closer to the body - proximal, the other is further away from it - distal. The segment of the tubular bone located between the diaphysis and the epiphysis is called metaphysis. The processes of bone that serve to attach muscles are called apophyses.
spongy bones are located in those parts of the skeleton where it is necessary to provide sufficient strength and support with a small range of motion. Among spongy bones, there are long(ribs, sternum) short(vertebrae, bones of the wrist, tarsus) and flat(bones of the skull, bones of the belts). Cancellous bones include sesamoid bones (patella, pisiform bone, sesamoid bones of fingers and toes). They are located near the joints, are not directly connected with the bones of the skeleton and develop in the thickness of the tendons of the muscles. The presence of these bones contributes to an increase in the arm of the muscle and, consequently, to an increase in its torque.
mixed dice- this includes bones that merge from several parts that have a different function, structure and development (bones of the base of the skull).